Research Library
The top resource for free research, white papers, reports, case studies, magazines, and eBooks.
- Agriculture
- Automotive
- Career
- Construction
- Education
- Engineering
- Broadcast Engineering
- Chemical
- Civil and Environmental
- Control Engineering
- Design Engineering
- Electrical Engineering
- GIS
- General Engineering
- Industrial Engineering
- Manufacturing Engineering
- Materials Science
- Mechanical Engineering
- Medical Devices
- Photonics
- Power Engineering
- Process Engineering
- Test and Measurement
- Finance
- Food and Beverage
- Government
- Healthcare and Medical
- Human Resources
- Information Technology
- Data Infrastructure
- Data Tools
- Desktops, Laptops and OS
- Chip Sets
- Collaboration Tools
- Desktop Systems - PCs
- Email Client
- Embedded Systems
- Hardware and Periferals
- Laptops
- Linux - Open Source
- Mac OS
- Memory Components
- Mobile Devices
- Presentation Software
- Processors
- Spreadsheets
- Thin Clients
- Upgrades and Migration
- Windows 7
- Windows Vista
- Windows XP
- Word Processing
- Workstations
- Enterprise Applications
- IT Infrastructure
- IT Management
- Networking and Communications
- Bluetooth
- DSL
- GPS
- GSM
- Industry Standard Protocols
- LAN - WAN
- Management
- Mobile - Wireless Communications
- Network
- Network Administration
- Network Design
- Network Disaster Recovery
- Network Interface Cards
- Network Operating Systems
- PBX
- RFID
- Scalability
- TCP - IP
- Telecom Hardware
- Telecom Regulation
- Telecom Services
- Telephony Architecture
- Unified Communications
- VPNs
- VoIP - IP Telephony
- Voice Mail
- WAP
- Wi-Fi (802.11)
- WiMAX (802.16)
- Wide Area Networks (WAN)
- Wireless Internet
- Wireless LAN
- Security
- Servers and Server OS
- Software and Web Development
- .Net Framework
- ASPs
- Application Development
- Application Servers
- Collaboration
- Component-Based
- Content Management
- E-Commerce - E-Business
- Enterprise Applications
- HTML
- IM
- IP Technologies
- Integration
- Internet
- Intranet
- J2EE
- Java
- Middleware
- Open Source
- Programming Languages
- Quality Assurance
- SAAS
- Service-Oriented Architecture (SOA)
- Software Engineering
- Software and Development
- Web Design
- Web Design and Development
- Web Development and Technology
- XML
- Storage
- Life Sciences
- Lifestyle
- Management
- Manufacturing
- Marketing
- Meetings and Travel
- Multimedia
- Operations
- Retail
- Sales
- Trade/Professional Services
- Utility and Energy
- View All Topics
- Featured eBooks
- Trending Resources
- New Resources
- Promote Your Content
- Partnership Opportunities
- Get RSS Updates
- About TradePub.com
- FAQ
- Contact Us
Share Your Content with Us
on TradePub.com for readers like you. LEARN MORE
Request Your Free Infographic Now:
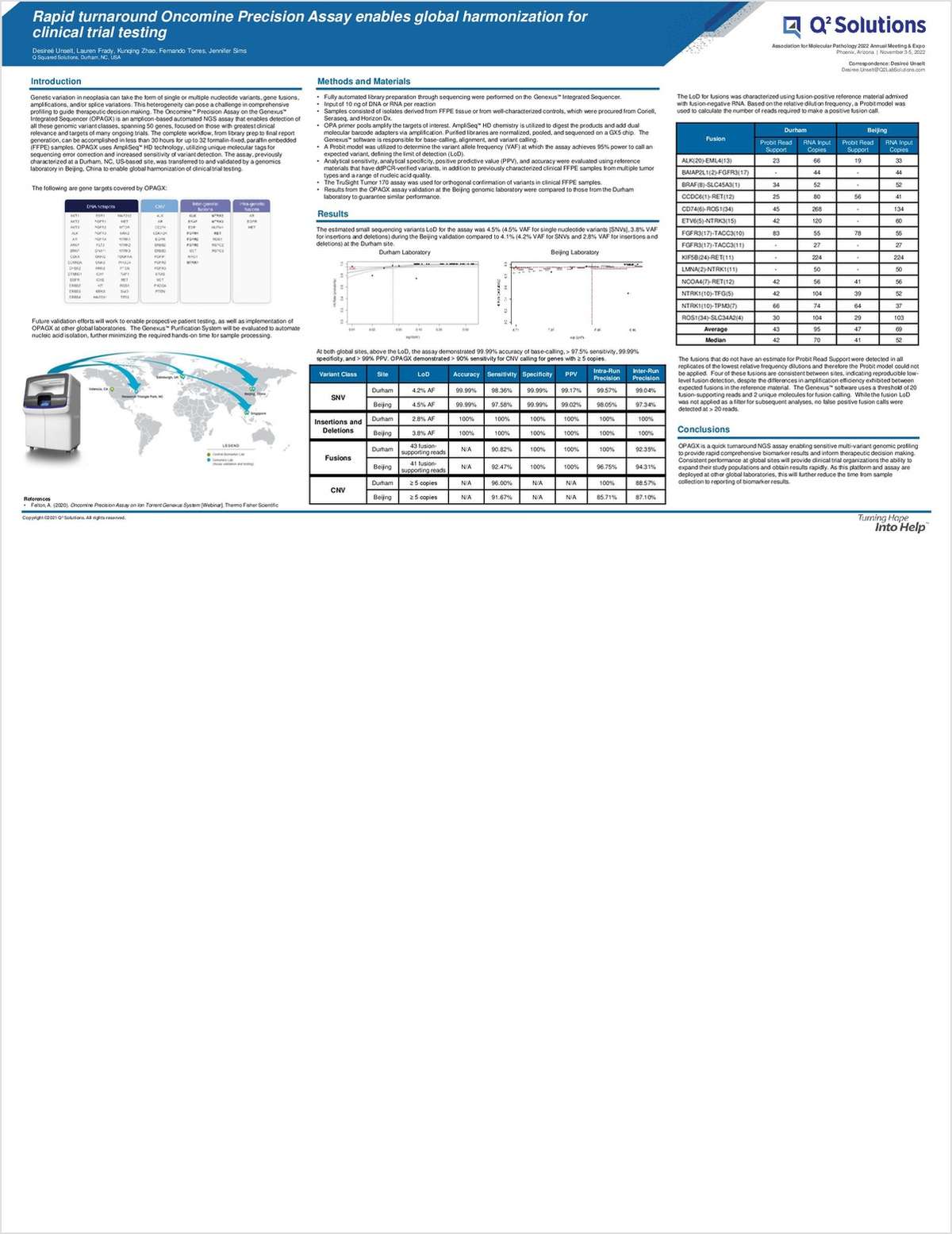
"Rapid-Turnaround Oncomine Precision Assay Enables Global Harmonization for Clinical Trial Testing"
Genetic variation in neoplasia can take the form of single or multiple nucleotide variants, gene fusions, amplifications, and/or splice variations. This heterogeneity can pose a challenge in comprehensive profiling to guide therapeutic decision making.
The Oncomine Precision Assay on the Genexus Integrated Sequencer is an amplicon-based automated NGS assay that enables the detection of all these genomic variant classes, spanning 50 genes, focused on those with the greatest clinical relevance and targets of many ongoing trials. The complete workflow, from library prep to final report generation, can be accomplished in less than 30 hours for up to 32 formalin-fixed paraffin-embedded samples. OPAGX uses AmpliSeq HD technology, utilizing unique molecular tags for sequencing error correction and increased sensitivity of variant detection.
This poster from Q2 Solutions describes a study in which the Oncomine Precision Assay on the Genexus Integrated Sequencer was characterized at a Durham, NC-based site and then transferred to and validated by a genomics laboratory in Beijing, enabling global harmonization of clinical trial testing.
Offered Free by: Q2 Solutions
See All Resources from: Q2 Solutions